What Is The Charge Of H2O
What Is The Charge Of H2O. Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste. An oxygen atom and two hydrogen atoms.
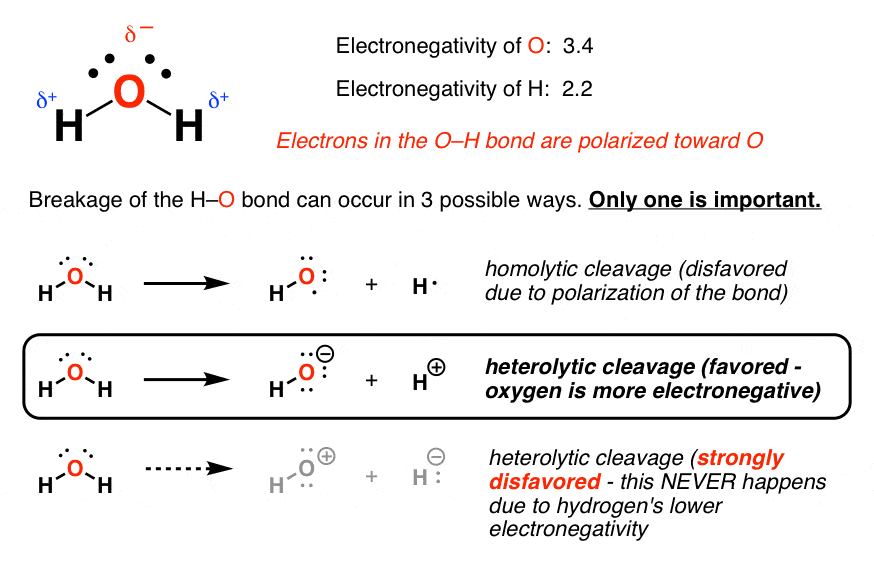
The oxygen atom has 8 protons and 8 electrons. In solution, the water molecule is conceived to dissociate, 2h 2o(l) ⇌ h 3o+ + −oh. On the right is an image of water's.
It Is Polar With The Oxygen Being Negative And The Hydrogen Positive.
Because the water molecule has the same number of protons as electrons, a water molecule is neutral. The formal charge on o atom of a h2o molecule is zero. What is the ionic charge of h2o?
However, The Uneven Distribution Of The Three Atoms Results.
A molecule of water is made up of three components: Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat. There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a.
Of Valance Elec… View The Full Answer
For example, sodium (na) and chlorine (cl) form an ionic bond to make nacl (table salt). Oxygen has eight protons and eight electrons. The oxygen atom has 8 protons and 8 electrons.
Since There Are 2 H Atoms So It Is 2X.
Ch4 + 2o2 co2 + h2o b. An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. There are 1 + 1 + 8 protonic charges, which are electrostatically balanced by 1 +1 +8 electronic charges.
What Do Well Call These Fundamental Carriers Of Electrostatic Charge, And Where Do They Live?
National center for biotechnology information. Small amounts of gaseous hydrogen peroxide occur naturally in the air. The oxygen atom forms two single sigma bonds with the hydrogen atoms in the h 2 o molecule.
Post a Comment for "What Is The Charge Of H2O"